Inflammatory bowel disease (IBD) is a group of life-long diseases affecting the intestines. The main types of IBD are ulcerative colitis (UC) and Crohn’s disease. More than three million people in the US have been diagnosed with IBD, with about 60% having ulcerative colitis and the remaining 40% with Crohn’s. What’s more, the incidence appears to be rising. Its more advanced form of moderate to severe UC causes inflammation in the colon, leading to ulcers in the lining of the large intestine.
Inflammatory bowel disease (IBD) is a group of life-long diseases affecting the intestines. The main types of IBD are ulcerative colitis (UC) and Crohn’s disease. More than three million people in the US have been diagnosed with IBD, with about 60% having ulcerative colitis and the remaining 40% with Crohn’s. What’s more, the incidence appears to be rising. Its more advanced form of moderate to severe UC causes inflammation in the colon, leading to ulcers in the lining of the large intestine.
Ulcerative colitis is a chronic condition that can cause recurring episodes of abdominal pain, bloody diarrhea, and weight loss, followed by periods of remission when symptoms subside. A recent study published in the Journal of American Medicine (JAMA – September 2024) investigated a new medication called risankizumab, marketed by Abbvie with the name Skyrizi, to see if it can help patients with moderate to severe UC achieve and maintain remission.
The JAMA study at a glance
Skyrizi (generic name risankizumab) is a biologic drug (a monoclonal antibody) that targets an inflammatory protein called interleukin-23 (IL-23) in the immune system. Since many patients with moderate to severe ulcerative colitis don’t respond to existing therapies (for example, about one-third don’t respond to first-line anti-TNF biologics, and many others lose response over time), researchers are studying new treatments like risankizumab that work differently. The JAMA article reported on two large clinical trials (called INSPIRE and COMMAND) that tested risankizumab in adults with moderately to severely active UC who had not improved with standard treatments.
- Induction Phase (INSPIRE) – In this 12-week trial, 975 patients were randomly split into two groups: two-thirds received risankizumab and one-third received a placebo (dummy treatment). Risankizumab was given as an intravenous (IV) infusion (1,200 mg dose) at weeks 0, 4, and 8, while the placebo group received IV infusions without active drug. The goal was to see how many patients could achieve clinical remission by 12 weeks – essentially, having minimal to no symptoms and a healed colon lining.
- Maintenance Phase (COMMAND) – Patients who improved on risankizumab in the first phase were then enrolled into a 40-week maintenance trial (weeks 12 to 52). Here, they were randomly assigned to one of three groups: 180 mg risankizumab, 360 mg risankizumab, or placebo, given as a subcutaneous injection (a shot under the skin) every 8 weeks. This tested whether continuing risankizumab could keep patients in remission for a full year, compared to stopping the drug (placebo group).
How well does risankizumab work for moderate to severe UC?

- Induction (12 weeks): About 20% of patients on risankizumab achieved clinical remission, compared to only 6% of those on placebo. In other words, risankizumab was over three times more effective than no treatment in inducing remission within three months. Patients on the drug also showed greater healing of the colon lining (measured by endoscopic improvement) than the placebo group. This means their inflammation and ulcers visibly improved on colonoscopy more often with the medication.
- Maintenance (1 year): Among patients who responded to initial risankizumab therapy, continuing the drug helped many stay in remission. At week 52, about 40% of patients on risankizumab (at either dose) were still in clinical remission, compared to 25% of those who were switched to placebo. Both doses (180 mg and 360 mg) performed similarly well, suggesting the lower dose was sufficient for most patients. By staying on risankizumab, patients had a higher likelihood of sustained symptom relief and colon healing over the year than those who stopped the medication.
Notably, the trials found that risankizumab’s effectiveness was somewhat influenced by patients’ prior treatment history. People who had never been on biologic drugs before tended to respond better than those who had previously tried and failed other biologics. In the maintenance study, over 50% of biologic-naïve patients maintained remission on risankizumab (versus around 31% on placebo), whereas remission rates were lower in patients who had past biologic treatments. This suggests that using risankizumab earlier in the treatment course (before multiple other drugs) might lead to better outcomes, though it can still help patients who have tried other therapies.
What this means for patients with moderate to severe UC
For patients with moderate to severe ulcerative colitis, these findings offer hope and a new treatment option. Risankizumab belongs to a newer class of medications targeting IL-23, which is different from older biologics like anti-TNF agents (e.g. infliximab) or anti-integrin therapy (vedolizumab). Having risankizumab available expands the menu of therapies doctors can use to treat UC. In practical terms:
- An option when others fail: If UC is not well-controlled with standard treatments (such as mesalamine, steroids, or older biologics), risankizumab could be an alternative to consider. The study shows it can induce remission even in patients who’ve had a hard time with other medications. This is encouraging for those who feel like they’re running out of options.
- Potential for first-line biologic: Doctors are also evaluating where risankizumab fits best in therapy. Some gastroenterologists may choose it earlier in treatment for suitable patients, for example, if the patient is newly starting biologic therapy, given its favorable results and safety profile. It has the convenience of subcutaneous injections for maintenance, which some patients prefer over frequent IV infusions. (In this study, the induction was IV, but after that, it was an injection every two months.)
- What remission means: Achieving remission with a drug like risankizumab can greatly improve quality of life. Remission means minimal or no diarrhea, no rectal bleeding, and healing of the colon’s lining on examination. While 20-40% remission rates may sound modest, remember that ulcerative colitis is a hard disease to treat. Even these percentages represent a significant improvement over placebo, and many other patients still benefited with partial improvement if not full remission.
Of course, it’s important to keep expectations realistic. Not everyone on risankizumab responded, and some patients in the trial did not maintain remission for the whole year. Ongoing research will show whether combining it with other therapies or using it for longer periods might help even more patients. The bottom line is that Skyrizi offers a promising new avenue for the treatment of patients with moderate to severe UC, and for them, it means more hope of finding a therapy that works for their condition.
Safety and potential risks
One reassuring finding of the JAMA study was that risankizumab appeared safe, with no major side effect differences compared to placebo. Throughout the trials, the overall rates of adverse events (like infections or other complications) were similar in the risankizumab and placebo groups:
- Infections: Because risankizumab calms part of the immune system, there may have been worry about infections. In these trials, serious infections (and even milder ones like colds) were not significantly more common in the risankizumab-treated patients than in those on placebo. There was no increase in issues like shingles (herpes zoster) either, which can sometimes be a concern with immune-modulating drugs.
- General side effects: No new or unexpected side effects emerged. Common side effects for similar biologic medications (reported in other studies or real-world use) can include upper respiratory tract infections, headache, fatigue, or injection-site reactions, but the key point is that no specific safety red flags were seen with risankizumab in this one-year study. This suggests it has a favorable safety profile for UC patients, at least in the medium term.
- Long-term considerations: Since ulcerative colitis is a lifelong disease, patients may need treatment for many years. The study tracked patients for one year, and while results were positive, the authors note that longer-term data are still needed . As more patients stay on risankizumab beyond a year, doctors will watch for any rare or delayed side effects that might not have appeared in the trial window. It’s also important to remember that any time the immune system is suppressed, there’s a theoretical risk of infections or other issues, so regular monitoring with your healthcare provider is wise.
In summary, Skyrizi was not only effective but also well-tolerated in the trials, which is good news for patients. It has been FDA-approved for UC based on these results, and it provides a new therapy that can be used with confidence that the benefits outweigh potential risks. Patients should have an open discussion with their gastroenterologist about whether this medication might be appropriate for their individual case, considering their disease severity, past treatment responses, and any health factors that could affect therapy choices.
Frequently Asked Questions about Ulcerative Colitis
What is ulcerative colitis, and what are its symptoms
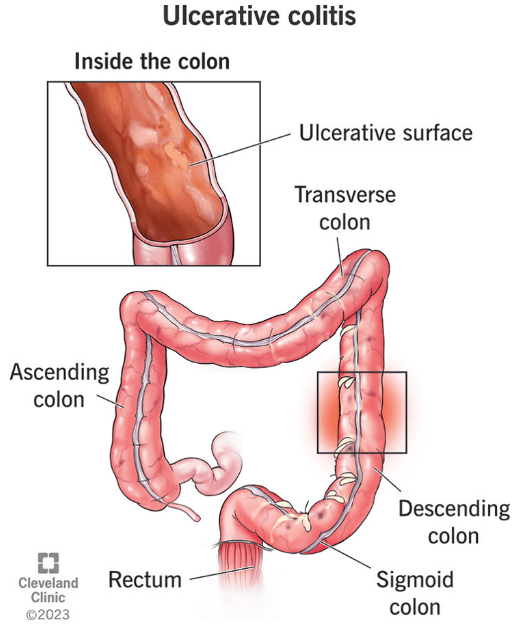
Ulcerative colitis (UC) is a long-term inflammatory disease of the colon (large intestine) in which the lining of the colon becomes inflamed and develops ulcers. This causes episodes of diarrhea (often with blood), abdominal cramping, rectal bleeding, and an urgent need to bowel movement; some people also lose weight or feel fatigued during flare-ups. The disease typically has ups and downs, with periods of active symptoms (flare-ups) followed by periods of little or no symptoms (remission).
What causes ulcerative colitis?
The exact cause isn’t fully understood, but ulcerative colitis is thought to be an autoimmune condition – the immune system mistakenly attacks the colon’s own cells, causing inflammation. Genetic factors play a role (having a family member with UC or Crohn’s raises risk), and differences in gut bacteria may contribute. Stress or certain foods do not cause UC, but they can trigger flare-ups or worsen symptoms in someone who already has the disease.
How is ulcerative colitis treated?
Treatment for UC focuses on reducing inflammation and achieving remission (relief of symptoms). Most patients take medications, which may include:
- Amino salicylates – anti-inflammatory drugs like Asacol, Pentasa, Canasa, and Mezavant, (all based on mesalamine) for mild cases
- Corticosteroids like Prednisone for short-term control of severe flares
- Immunomodulators (such as Imuran or Neoral) to suppress immune activity
- Biologic therapies like Skyrizi, Remicade, or newer agents like Stelara that target specific immune pathways.
These medicines help heal the colon and reduce symptoms. If medications don’t work or if complications arise, surgery to remove the colon and rectum (proctocolectomy) can cure ulcerative colitis by eliminating the diseased organ. Surgery is usually a last resort but can be life-saving or curative, essentially ending the colitis, although it means living with an internal pouch or ileostomy.
Is ulcerative colitis curable, or does it ever go away?
Ulcerative colitis is chronic, meaning it usually does not go away completely by itself. The only definitive cure is to surgically remove the colon and rectum, which permanently eliminates the disease. However, many people manage UC with medications and achieve long stretches of remission where they have no symptoms. In remission, a patient might feel completely well for months or years, but there is always a possibility of flares returning since the underlying tendency for inflammation remains. The goal of therapy is to maintain remission as long as possible; with the right treatment plan, a person with UC can often lead a normal, active life.
Can diet help with ulcerative colitis, and are there foods to avoid?
Diet alone cannot cure or cause ulcerative colitis, but it can influence the patient’s comfort and symptom control. Certain foods can make symptoms worse during a flare-up. Common triggers include dairy products (for some people, especially if lactose intolerant), high-fiber foods like nuts, corn, raw vegetables, and whole grains (these can aggravate diarrhea during flares), as well as spicy or fatty foods. Beverages like alcohol and caffeine can also irritate the gut and should be limited if they worsen symptoms. It’s a good idea to keep a food diary to identify which foods you are sensitive to since triggers can vary from person to person. During active flares, doctors often recommend a bland, low-fiber diet to ease symptoms, then a balanced, healthy diet when in remission. Remember, maintaining good nutrition is important since chronic diarrhea and poor intake can lead to deficiencies.
What is the difference between ulcerative colitis and Crohn’s Disease?
Ulcerative colitis and Crohn’s Disease are the two main types of inflammatory bowel disease (IBD). The key difference is the location and pattern of inflammation. Ulcerative colitis only affects the colon (large intestine), usually starting at the rectum and extending continuously through part or all of the colon, and it inflames only the inner lining of the bowel Moderate to severe UC can also cause ulcers. In contrast, Crohn’s disease can affect any part of the digestive tract from the mouth to the anus, and it often occurs in patchy areas (with healthy tissue between inflamed sections). Crohn’s inflammation can also go deeper into the intestinal wall, sometimes causing complications like fistulas (abnormal connections) or strictures, which are less common in UC. In short, UC affects only the colon and causes continuous surface inflammation, while Crohn’s affects anywhere in the gut with skip lesions and deeper inflammation.