I spend a fair bit of time reviewing and replying to questions and requests from our customers looking for information and guidance specifically about the explosion of exciting new treatments that are being built around the great breakthrough GLP-1 drugs, and I know that most people have become familiar with this subject in the areas of diabetes and weight-loss treatment. But I’m guessing that for most people without technical training, seeing them now labelled as “cardioprotective” (meaning they protect the heart) is a big leap. The question I reviewed takes us up to an even higher plane, talking about dementia.
Even labeling glucose-lowering agents as being inherently cardioprotective may be something entirely new for most people who have only recently become aware of the main features of GLP-1 glucose-lowering medications. Then, it’s another big step to get from a medication that’s only recently been recognized as benefiting some organ-related conditions (in this case, the heart and pancreas) and elevating that up to behavior and cognitive function. So, I am going to take a step back and review the remarkable progression that has occurred in the very short space of time since drugs like Ozempic, Mounjaro and Wegovy hit our shelves and then look at how these drugs might reshape how we think about aging, brain health, and preventive medicine more broadly. And that’s one hell of a plot twist.
By now, you’ve probably heard that semaglutide and tirzepatide, the substances that Ozempic/Wegovy and Mounjaro are based on, aren’t just other forms of diabetes medication. That’s old news. Instead, they are blockbusters that we’ve spoken about a few times, for example, here and here. They produced a weight-loss revelation. Now they are turning out to be a cardiovascular ally, and apparently, they are being positioned as the brain’s new best friend.
But let’s take a moment and unpack how we got here, because while GLP-1 RAs began as a specialized drug class for type 2 diabetes, they are now being roped into conversations about subjects as varied as heart and liver health, behavior modification, and dementia prevention. And this is not happening in a speculative “maybe one day” kind of way. No, we’re talking real, statistically significant results from randomized trials pointing toward GLP-1 receptor agonists possibly being neuroprotective. Wild, right?
Let’s review the programme for the show.
Overture – GLP-1 treats blood sugar problems
The original purpose of GLP-1 was the taming of type 2 diabetes.
GLP-1 receptor agonists (GLP-1 RAs) like semaglutide (Ozempic) and tirzepatide (Mounjaro), and earlier forms like liraglutide and dulaglutide were initially engineered to mimic a natural gut hormone, glucagon-like peptide-1. In simple terms, when blood sugar goes up after a meal, natural GLP-1 tells the pancreas to release some insulin. When the body either does not produce enough insulin on its own or fails to metabolize it quickly enough, the drugs do the same thing, helping control post-meal blood sugar spikes in type 2 diabetes.
 Act I – Enter the hero
Act I – Enter the hero
And here’s the kicker. GLP-1 RAs also delay gastric emptying and suppress appetite. That second effect is what led to their dramatic impact on weight loss, eventually culminating in FDA approval of Wegovy (also based on semaglutide) as an obesity treatment. And once people started shedding serious pounds with these drugs, the media and the wider public took notice.
Act II – A cardiovascular plot twist
After just a few years of widespread use of GLP-1 RAs, it was found that losing weight isn’t the only bonus. A series of large, well-designed clinical trials showed that GLP-1 RAs reduced major cardiovascular events, like heart attacks and strokes, and reduced the incidence of cardiovascular catastrophes in people with type 2 diabetes. That was not trivial. That is a seismic shift. So much so that the American Diabetes Association and European Society of Cardiology (Source: Cosentino F, Grant PJ, Aboyans V, et al. 2023 ESC Guidelines on diabetes, pre-diabetes and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2023;44(28):2373–2474. DOI: 10.1093/eurheartj/ehad294) now recommend GLP-1 RAs as first-line therapy for many diabetic patients with cardiovascular disease, even before considering other traditional glucose-lowering agents.
And then things got even stranger.
Act III: Entering left – the brain takes center stage

People on these medications are reporting a drop in gambling, drinking, smoking, drug use, and even compulsive shopping. That’s not a side effect; that’s a behavior-altering phenomenon. As Dr. Perry Wilson put it: “It’s hard to find an organ system or self-destructive behavior that GLP-1 drugs don’t seem to improve somehow”.
It’s not just hearsay anymore. The JAMA meta-analysis that led me into this subject pooled data from 23 randomized trials with over 160,000 participants. They asked the question that set me off on this subject: Are glucose-lowering agents based on GLP-1 RAs associated with reduced risk of dementia or cognitive impairment?
The results?
Quoting the JAMA paper, the GLP-1 RA drugs showed a statistically significant benefit. Odds Ratio for dementia: 0.55 (95% CI, 0.35–0.86).
A short digression – what does “Odds Ratio” mean
An odds ratio (OR) is a way researchers compare how likely something is to happen in one group versus another.
Think of it like this:
🔹 If the odds ratio is 1, it means there’s no difference between the two groups.
🔹 If the odds ratio is less than 1, the event is less likely to happen in the group getting the treatment.
🔹 If the odds ratio is more than 1, the event is more likely in the treatment group.
In the study on GLP-1 drugs and dementia, the odds ratio was 0.55 for people who took GLP-1 medications compared to those who didn’t. So that means the people taking GLP-1 drugs had about a 45% lower chance of being diagnosed with dementia compared to those taking a placebo. Another way to put it – if 100 people on placebo were diagnosed with dementia, only about 55 people (or fewer) would be expected to have developed it in the GLP-1 group, all else being equal.
Let that sink in—a 45% reduction in dementia risk in the treatment group.
Other drugs that also function to control blood sugar levels, such as SGLT2 inhibitors and pioglitazone, didn’t show the same effect. Not even close! SGLT2 inhibitors, despite their cardiovascular benefits, had an odds ratio of 1.20 (i.e., no protection, maybe even harm, though the confidence intervals were wide).
Intermezzo – So… what’s all the buzz about?
For GLP-1 molecules to do their work, they need to find receptors in locations around the body into which they can plug and kick off a metabolic process. To answer that, Dr. Wilson gives us a clue that’s both obvious and overlooked: look at where the GLP-1 receptors are found in the body.
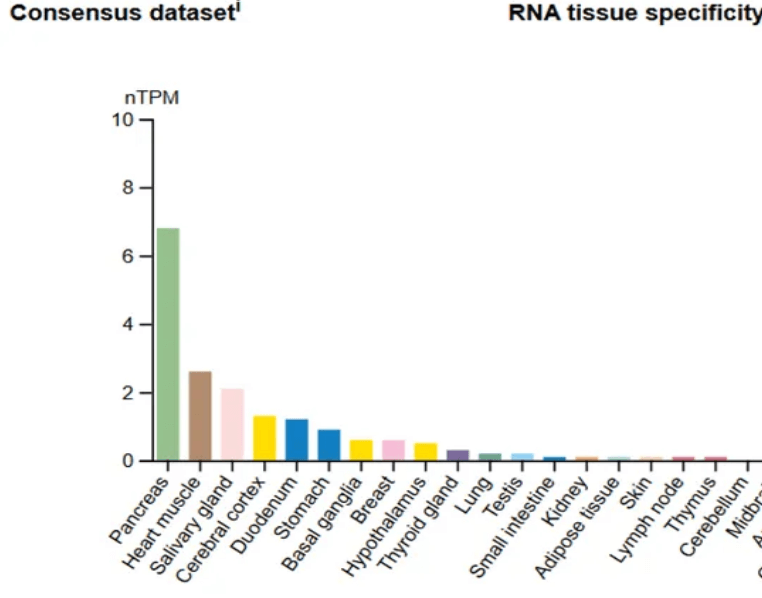
- The pancreas (no surprise)
- The heart muscle (explaining those newly observed cardioprotective effects)
- The food-processing organs (from the mouth on down)
- Interestingly, the cerebral cortex is involved specifically in areas of thinking, memory, and decision-making.
So now we’re seeing:
- A drug class that binds to brain receptors
- Documented reductions in self-destructive behaviors
- And emerging evidence that it reduces dementia risk.
This isn’t just about blood sugar anymore.
Interval – Hear what the critics have to say
Now, before we crow that semaglutide is the brain’s new “miracle drug”, it’s necessary to pay attention to some of the fine print of the JAMA meta-analysis:
- None of the trials included in the meta-analysis was designed with dementia as the primary outcome. That means dementia data was often reported as an adverse event, and not a rigorously tracked endpoint.
- The total dementia numbers reported were low. Many trials had just a few cases of dementia in each arm. For example, the HARMONY trial had zero dementia cases in the albiglutide arm and three in the placebo. Not exactly blockbuster stats, unless you stitch together a dozen trials via meta-analysis. To refute that critique, that’s exactly what this paper did – it’s a meta-analysis of 30+ different studies, giving it a much higher degree of reliability.
- No metformin trials were included. This is frustrating because metformin has long been rumored to have neuroprotective effects, but the data couldn’t be included because it was too inconsistent.
Still, the meta-analytic power matters. When you combine small signals across many well-run studies, you reduce noise and amplify patterns. And here, that pattern is real: GLP-1s help the brain.
Act IV: Type 3 diabetes pulls into town
While everyone is familiar with the distinctions between type 1 and type 2 diabetes, there’s now at least one new form of insulin resistance (type 3), and there may even be another. For now, let’s concentrate on type 3, for which there is more solid research and agreement. The alternative name for type 3 diabetes is Alzheimer’s disease – yes … that one!
The term type 3 diabetes has been proposed to describe Alzheimer’s disease due to shared characteristics in the brain with Type 2 diabetes, notably insulin resistance and impaired glucose metabolism. In Alzheimer’s disease (AD), neurons exhibit reduced responsiveness to insulin, leading to decreased glucose uptake, energy deficits, and subsequent neurodegeneration. This insulin resistance is associated with the distinctive AD pathologies, including amyloid-beta accumulation, tau protein hyperphosphorylation, and oxidative stress.
GLP-1 RAs have demonstrated potential neuroprotective effects in AD. They can cross the blood-brain barrier and bind to GLP-1 receptors expressed on various brain cells. Activation of these receptors may:
- Enhance insulin signaling in the brain, improving neuronal glucose uptake and metabolism.
- Reduce neuroinflammation by modulating microglial activation and cytokine release.
- Decrease amyloid-beta and tau pathologies, mitigating plaque formation and neurofibrillary tangles.
- Promote neurogenesis and synaptic plasticity, supporting cognitive functions.
Clinical studies have begun to explore these effects. A Phase 2b trial reported that patients with mild AD receiving liraglutide experienced an 18% slower cognitive decline and 50% less brain atrophy in memory-related regions compared to placebo. Additionally, observational data suggest that semaglutide use in individuals with Type 2 diabetes is associated with a 40–70% reduced risk of developing AD.
Ongoing phase 3 trials, such as EVOKE and EVOKE Plus, are evaluating how well semaglutide works in early-stage AD, with results anticipated soon. If successful, GLP-1 RAs could represent a novel therapeutic strategy targeting the metabolic dysfunctions underlying AD, offering hope for disease modification in this challenging neurodegenerative condition.
Act V: Introduce the weight-dementia hypothesis
There’s another potential outlier here. GLP-1 drugs induce profound weight loss, unlike SGLT2 inhibitors. Could the benefit to brain health be driven not just by blood sugar control, but by weight loss itself? After all, obesity is a known risk factor for dementia. So this becomes an elegant hypothesis: maybe GLP-1 RAs prevent cognitive decline by lowering inflammation, reducing insulin resistance, and slimming the waistline, all at once.
Currently planned studies, including EVOKE and Oxford Semaglutide and Cognition (OxSENSE), are designed to specifically track cognitive outcomes. They are designed to tell us whether GLP-1s can prevent dementia in people without diabetes, which would be the ultimate plot twist.
Finale: What does this mean for the future?

And that’s not just a win for patients. It’s a winning goal, a hole-in-one moment for medicine, especially when you consider that until now, the pharmacological response to Alzheimer’s has been less than satisfactory.
FAQs about the new features of GLP-1 receptor agonist drugs
Where did the data that GLP-1 drugs like Ozempic may reduce the risk of dementia come from?
Emerging research suggests that GLP-1 receptor agonists (GLP-1 RAs) may indeed produce a reduced risk of dementia, particularly all-cause dementia. The article that started me off on this topic was a 2025 JAMA Neurology meta-analysis that pooled data from over 160,000 participants, which found a 45% reduction in the odds of developing dementia among people randomized to receive GLP-1 RAs versus placebo. Importantly, this benefit was not seen with other diabetes medications like SGLT2 inhibitors or pioglitazone. While the individual trials weren’t specifically designed to assess dementia as a primary outcome, the pattern across multiple studies points to a genuine neuroprotective effect. Researchers speculate this may be due to GLP-1 receptors being expressed in the cerebral cortex, the part of the brain responsible for memory and decision-making. The drugs may also reduce inflammation, improve blood flow, and lower insulin resistance, all factors implicated in cognitive decline. While these findings are promising, dedicated trials such as EVOKE and OxSense are currently underway to confirm whether GLP-1 drugs can prevent dementia in people without diabetes.
Do GLP-1 medications affect addictive behaviors like smoking or gambling?
GLP-1 receptor agonists appear to influence parts of the brain involved in reward, impulse control, and decision-making, which may explain their surprising impact on addictive behaviors. Multiple anecdotal reports and early observational studies suggest that people taking drugs like semaglutide or tirzepatide experience less urge to drink alcohol, smoke, gamble, or engage in compulsive shopping. The mechanism isn’t fully understood, but one key clue lies in where GLP-1 receptors are found. According to Protein Atlas data, these receptors are present not just in the pancreas and heart but also in the cerebral cortex, including regions tied to reward pathways. Animal models have also shown GLP-1 agonists reduce behaviors like cocaine-seeking and alcohol consumption. One hypothesis is that these drugs dampen dopaminergic drive, which reduces the “hit” people get from risky or pleasurable behaviors. While there aren’t yet any randomized trials designed specifically to study addiction outcomes, the off-target behavioral changes are consistent enough to warrant further investigation. This could signal a potential new role for GLP-1 drugs in treating addiction.
Do GLP-1 drugs provide benefits beyond weight loss and blood sugar control?
Yes, GLP-1 receptor agonists now occupy the pinnacle of a rapidly evolving therapeutic landscape. Originally developed to improve blood glucose control in people with type 2 diabetes, these drugs have since shown strong effects on weight loss, leading to FDA approval for obesity treatment (Wegovy). But the benefits go well beyond that. Clinical trials have demonstrated that GLP-1 drugs can significantly reduce cardiovascular events, including heart attacks and strokes, particularly in patients with type 2 diabetes and pre-existing heart disease. There is also growing evidence that they may improve liver function in people with non-alcoholic fatty liver disease (NAFLD) or metabolic dysfunction-associated steatohepatitis (MASH). Recently, they’ve been linked to neuroprotective effects, including reduced risk of dementia. Some observational reports even suggest improvements in inflammation markers, kidney function, and mental health. Though not all these effects are fully understood, they appear to stem from a mix of direct receptor action and indirect benefits such as weight loss, improved insulin sensitivity, and reduced systemic inflammation. It’s no exaggeration to say GLP-1s are evolving into multi-system therapeutics.
Are GLP-1 receptor agonists being studied for use other than for people with diabetes?
Yes, and this is one of the most exciting developments in the GLP-1 environment. Several GLP-1 receptor agonists, including semaglutide and tirzepatide, are already approved for use in obese individuals without diabetes, thanks to their profound weight-reduction effects. But researchers are now pushing even further. Trials like EVOKE and OxSense are exploring whether GLP-1 drugs can prevent or slow the progression of cognitive decline in non-diabetic populations. There are also early studies investigating their impact on addiction, anxiety, cardiovascular health, and non-alcoholic liver disease in patients without diabetes. These studies aim to determine whether the effects seen in diabetic populations can be replicated, or even enhanced, in broader groups. In short, GLP-1 drugs are no longer just “diabetes meds”, they’re being tested as metabolic, neurological, and behavioral modulators, with the potential to reshape chronic disease management for millions.
What are the limitations of current GLP-1 studies in terms of any reduced risk of dementia?
While the emerging data on GLP-1 drugs and brain health is compelling, there are still important limitations to keep in mind. First, most existing trials were not designed with dementia or behavioral changes as a primary endpoint. In many cases, dementia was only recorded as an adverse event, which introduces potential for underreporting or misclassification. Second, the number of dementia events in these studies was very small, often just a few per treatment arm, making the conclusions statistically fragile unless pooled in a meta-analysis. This was why the study that started me off on this subject was so compelling, because it did just that – a meta-analysis of dozens of studies that involved over 160,000 subjects.
As well, the period of the study was relatively short (stated as being around 31 months) and while GLP-1s appear to impact addictive behaviors, there’s a lack of formal behavioral trials to validate and explain these effects. All told, these limitations underscore the need for purpose-built, longer-term studies to fully understand GLP-1s’ neurocognitive potential, which I look forward to being able to report to you in due course.
What is “Type 3 diabetes,” and how is it connected to Alzheimer’s disease?
“Type 3 diabetes” is an informal but increasingly used term to describe Alzheimer’s disease (AD) as a form of brain-specific insulin resistance. In this framework, the brain’s cells, particularly neurons, become less responsive to insulin, leading to impaired glucose uptake, energy shortages, and eventually, neurodegeneration. This is similar to what happens in Type 2 diabetes, but the damage is localized to the brain. Studies have shown that people with Type 2 diabetes have a significantly higher risk of developing Alzheimer’s, and insulin resistance is now recognized as a major contributor to amyloid plaque buildup, tau tangles, oxidative stress, and inflammation, all hallmarks of Alzheimer’s pathology. This has led researchers to explore drugs originally developed for diabetes, especially GLP-1 receptor agonists, as potential treatments for neurodegenerative diseases. GLP-1s are of particular interest because they cross the blood-brain barrier and act on GLP-1 receptors found in memory-critical regions like the hippocampus and cerebral cortex, areas heavily affected in Alzheimer’s disease.
Are GLP-1 drugs like semaglutide being tested as treatments for Alzheimer’s?
Yes — and the results are encouraging so far. GLP-1 receptor agonists, especially semaglutide (Ozempic, Wegovy) and liraglutide (Victoza), are currently being studied in clinical trials for their potential to slow or prevent cognitive decline. In lab models, these drugs have been shown they reduce brain inflammation, lower levels of amyloid-beta and tau proteins (which are central to Alzheimer’s pathology), and even promote the growth of new brain cells. A Phase 2 trial of liraglutide showed slower memory loss and reduced brain atrophy in Alzheimer’s patients. Meanwhile, semaglutide is being tested in two large Phase 3 trials – EVOKE and EVOKE Plus – targeting early-stage Alzheimer’s disease, with results expected soon. These studies are the first to directly evaluate whether a GLP-1 drug can delay or prevent Alzheimer’s in people who do not have diabetes. If successful, this would represent a groundbreaking shift — using a metabolic drug to target the neurological dysfunction at the root of Alzheimer’s, potentially redefining how we treat or even prevent this devastating condition.

 Act I – Enter the hero
Act I – Enter the hero